The Hand Warmer Design Challenge Investigation 12 CHALLENGE The ideal hand warmer increases in temperature by 20C but no more as quickly as possible has a volume of about 50 mL costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as possible. To activate your newest most innovative LSDH hand warmer yet all you have to do is shake it.

Solid Important Notes From Msds Nacl Cacl 2 Nac 2 H 3 O 2 Na 2 Co 3 Licl 2 Cost Course Hero
Roberts AP Chemistry Periods 2-3 The Hand Warmer Design Challenge Procedure.

. The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch. The Hand Warmer Design Challenge. Up to 24 cash back Designing a Hand Warmer Lab Introduction.
The goal of this lab is to design a safe effective environmentally benign and inexpensive hand warmer that will increase the temperature of water by 20 C but no more as quickly as possible with a volume of about 50 mL. Write down the procedure you will follow. Where Does the Heat Come From.
Chemistry Components Types and Terminology offers to the reader a clear and concise explanation of how Li-ion batteries are designed from the perspective of a manager sales person product manager or entry level engineer who is not already an expert in Li-ion battery design. Measure heat transfer using a calorimetry investigate energy changes accompanying the formation of substances and design a hand warmer that is reliable safe and inexpensive. The temperature of the water was measured and recorded.
DAY 1 Part 2 only. The Handbook of Lithium-Ion Battery Pack Design. The Chemistry Of Hand Warmers.
DECEMBER 15 2010 Tis the season for cold fingers. In the experiment we used three types of Chemicals. If youre stuck out in the cold for a few hours your mittens can only do so much.
Working in groups of four each student group will be. Looking at the results we saw which chemicals created more amount of heat when its being applied to water and salt. CaCl2 Calcium Chloride MgSO4 Magnesium Sulfate and LiCl Lithium Chloride.
Designing A Hand Warmer Ap Lab Answers. The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer and the spontaneous direction of the transfer is always from a. The results provide a model for the guided-inquiry challenge which is to design an optimum hand warmer for consumer applications.
Explain it to your instructor before you proceed. Answer these in your lab notebook after the Purpose section 1 When chromium. When sharing data be sure you only add data for your group number and do not overwrite existing data.
However we are committed to providing the best of the best to our customers lets jump into it our family and. Learn Something New Every Day. Design an effective safe environmentally benign and inexpensive hand warmer.
The hand warmer you are designing needs to increase in temperature by 20 C. 48C - 245C 235C. If you would like to get the hand warmer design challenge pre lab answers might you have been looking for it in diverse online shops.
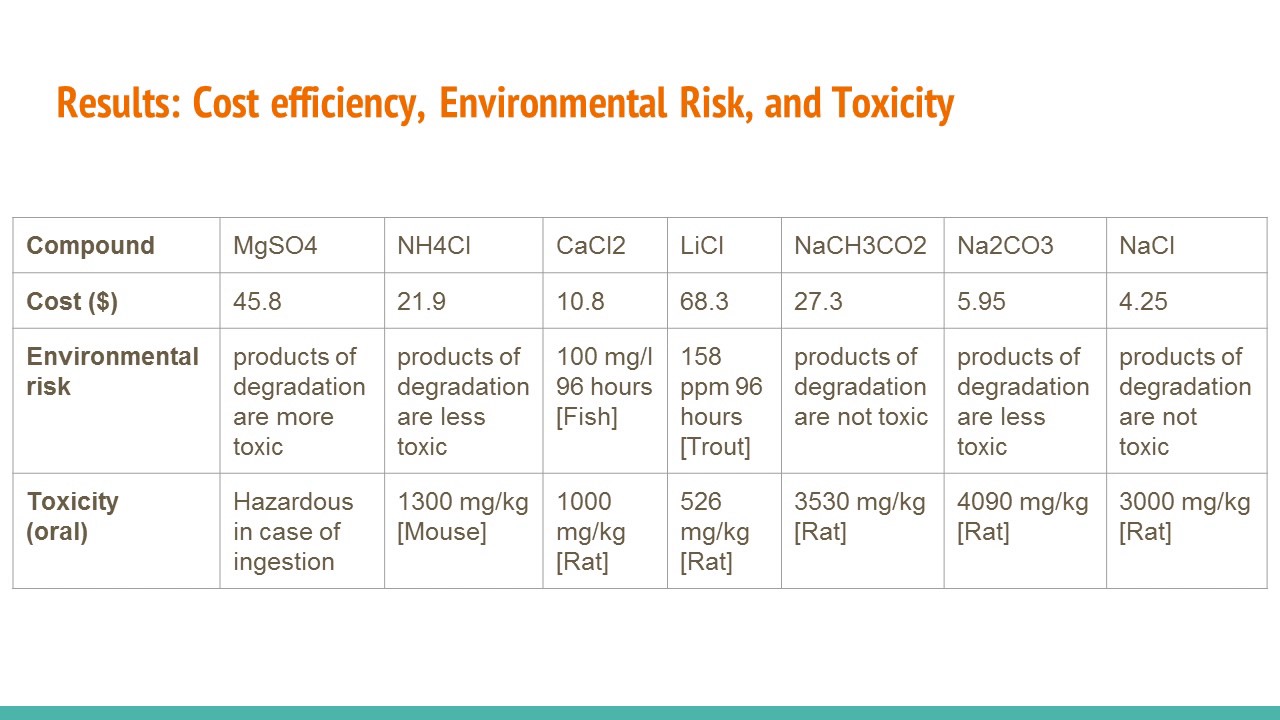
Environmental issues What environmental factors were considered 5. Up to 24 cash back Conclusion - Hand warmer challenge. Up to 24 cash back 1.
You will carry out an experiment to determine which substances in what. 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top wait 15 seconds. This type of hand warmer tends to produce a more vigorous heat than the dry powder type of hand warmer but does not produce heat for quite as long.
Up to 24 cash back Temperature Change T. For each solid consider safety cost and environmental impact as well as the amount of heat released or absorbed. Where Does the Heat Come From.
Up to 24 cash back suitable for use in a hand warmer. Hand Warmer Design Challenge Group Assignments Data Compilation Please make a note of your group number in your laboratory report. 233 would be required for a hand warmer that meets this requirement.
1 Measure out 2 separate samples of 1000 mL of distilled water. Review the criteria for an ideal hand warmer from the Central Challenge. Find the training resources you need for all your activities.
Hand warmer material choices Which three compounds were considered for your hand warmers 3. Of data gathered during experimental design 4. Up to 24 cash back The Hand Warmer Design Challenge.
AP MANUAL 2013 p. We are delighted to assist in getting the best the hand warmer design challenge pre lab answers thats fulfilling your desires. The salt dissolves and the water warms.
Hand Warmer Design Challenge Lab How It Works This super official graph shows CaCl2 produces more heat than any other substance. Download Free Designing A Hand Warmer Ap Lab Answers The Hand Warmer Design Challenge by Jason Santana designing a hand warmer ap lab answers - Bing OCOOPA Fast-Charging Hand Warmers 10000mAh Handwarmer with PD QC 30 Rechargeable Hand Warmer Supercar Design Heating time 15 Hrs Perfect for Outdoor. Experimental design How did you group test chemicals used for the hand warmer challenge 2.
Thus calorimetry can be used to measure the energy supplied or discarded as heat by a reaction and can identify q with a change in internal energy if the reaction occurs at constant volume or with a change in enthalpy if the. This needs to be included in your post. According to the lab we can see that the amount of salt in grams can make a difference like if we add more grams then it could probably change it a little higher.
Where Does the Heat Come From. Our hand warmer was an exothermic reaction since it released heat. Energyenthalpy change with process of dissolving a solute in solvent.
An ideal hand warmer increases in temperature by 20 C but no more as quickly as possible has a volume of about 50 mL costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as possible. Our top scientists use water and calcium chloride to create the most innovative design of hand warmers yet. Science In Your Mittens.
Q -786592 J Exothermic Reaction. Heat of Solution measures. Data collected Graphs tables etc.
The ideal hand warmer increases in temperature by 20C but no more as quickly as possible has a volume of about 50 mL costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as possible. Up to 24 cash back The Hand Warmer Design Challenge. Up to 24 cash back What we were doing was finding the right salt to mix it with water at room temperature and see the reactions and see which one can be best to use as a head warmer for cold places.
You should select a single person from the group to enter the data online to prevent confusion. Are the dissolving processes you carried out endothermic or exothermic or neither. A type of reaction where energy is released in the form of light or heat.
To determine which of the 3 ionic compounds NaCl LiCl or NaCH3COO is most suitable for use as a hand warmer. CENTRAL CHALLENGE The ideal hand warmer increases in temperature by 200C but no more as quickly as possible has a volume of about 50 mL costs as little as possible to make and uses chemicals that are as safe and environmentally friendly as possible. The calorimeter was assembled on a magnetic stirrer and 1000 mL of water was measured in a graduated cylinder and poured into the calorimeter.

Designing A Hand Warmer By Makayla Sabo

Ap Inquiry 12 Hand Warmer Challenge

Ap Chemistry Hand Warmer Lab Youtube

Hand Warmer Design Challenge Bremen High School District 228
Designing A Hand Warmer Lab Youtube



0 comments
Post a Comment